Significance
Tissue engineering scaffolds for bone regeneration provide a temporary, three-dimensional structure that supports osteoblast differentiation and formation of new bone tissue. The development and implementation of tissue engineering scaffolds for bone regeneration, despite their potential, face several challenges which researchers and clinicians try to overcome to advance scaffold-based approaches for bone regeneration. For instance, finding materials that are biocompatible and do not provoke an adverse immune response remains a challenge. Moreover, matching a scaffold’s degradation rate with the rate of new bone formation is critical-if a scaffold degrades too quickly, it may not provide sufficient support for bone growth while too slow a rate of degradation can limit the integration of new bone tissue. Furthermore, methods are needed to actively induce resident stem cells to differentiate into bone-forming cells. Addressing these challenges requires extensive research and innovation in materials science, molecular biology, and engineering. A new study published in Acta Biomaterialia and conducted by Chunxi Ge, Yiming Li, Fashuai Wu, Peter Ma, and led by Professor Renny Franceschi from the University of Michigan School of Dentistry, describes a new approach for activating the osteoblast differentiation of skeletal progenitor cells involving stimulation of the two main collagen receptors in bone, discoidin domain receptor 2 (DDR2) and collagen-binding integrins, using receptor-specific triple-helical peptides. The selective activation of these receptors by their respective peptides, GVMGFO for DDRs and GFOGER for integrins, provided insight into the molecular mechanisms underlying bone formation and also suggested a novel strategy for the development of tissue engineering scaffolds aimed at enhancing bone repair and regeneration.
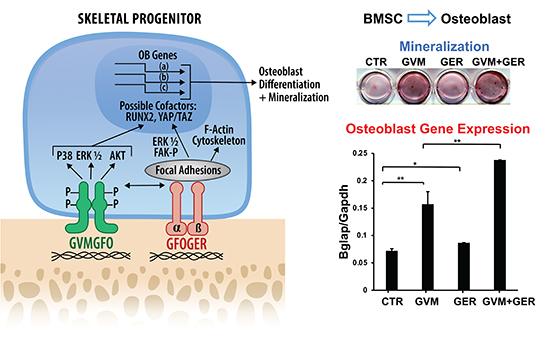
The team began by synthesizing two collagen mimetic triple-helical peptides; one containing the DDR2-binding sequence GVMGFO (termed GVM), and the other containing the integrin-binding sequence GFOGER (termed GER). To create a surface that mimics the extracellular matrix interactions with cells, the researchers coated tissue culture plates with these peptides or with type I collagen as a control. Cellular responses to peptides were assessed using a preosteoblast cell line (MC3T3-E1 cells), murine bone marrow stromal cells (BMSCs), and primary calvarial osteoblasts from Ddr2 flox/flox mice. The authors showed that the GVM peptide stimulated DDR2 phosphorylation at tyrosine 740 (Y740), a marker of DDR2 activation, without affecting integrin signaling while the GER peptide activated focal adhesion kinase (FAK) phosphorylation at tyrosine 397 (Y397), indicative of integrin activation, without influencing DDR2 phosphorylation. Remarkably, the combined application of GVM and GER peptides led to enhanced activation of both DDR2 and FAK/integrin pathways and significantly enhanced osteoblast differentiation. This synergistic effect was blocked in Ddr2-deficient cells, indicating DDR2’s essential role. Moreover, they used quantitative real-time PCR to measure the expression levels of osteoblast differentiation markers, including alkaline phosphatase, osteocalcin, and bone sialoprotein, revealing that peptide treatments modulate these critical markers. They also assessed the degree of mineralization which is a hallmark of osteoblast maturation using Alizarin Red staining. The combined peptides markedly enhanced mineral deposition compared to individual peptides or untreated controls.
According to the authors, the GVM and GER peptides selectively activated DDR2 and integrin pathways, respectively, with the GVM peptide notably stimulating osteoblast differentiation. However together, these peptides synergistically enhanced both DDR2 and integrin signaling, significantly boosting osteoblast differentiation beyond the effects observed with individual peptide treatments. Additionally, the cooperative effect on osteoblast differentiation was dependent on DDR2, which highlights its central role in mediating the synergistic enhancement of bone regeneration by these peptides. The authors’ findings suggest a novel approach for bone regeneration by combining DDR and integrin-activating peptides to mimic signaling cues of the natural extracellular matrix, which potentially may lead to improved outcomes in bone repair and tissue engineering scaffolds. In conclusion, the study by Professor Renny Franceschi and colleagues is an important step towards understanding and harnessing the molecular mechanisms of bone regeneration. Their finding has profound implications for the development of biomaterials for bone repair. The design of tissue engineering scaffolds incorporating both DDR and integrin-activating peptides could mimic the complex extracellular matrix environment, providing the structural support and also biochemical cues for optimal cell function. These scaffolds could potentially accelerate bone healing, and offer new solutions for bone loss resulting from trauma, surgical resections, or pathological conditions. In a statement to Medicine Innovates, Professor Renny Franceschi said “By mimicking signals normally provided to bone cells from the collagenous extracellular niche, this study points to a new strategy for bone tissue engineering involving coupling collagen mimetic peptides to synthetic scaffolds.”
Reference
Ge C, Li Y, Wu F, Ma P, Franceschi RT. Synthetic peptides activating discoidin domain receptor 2 and collagen-binding integrins cooperate to stimulate osteoblast differentiation of skeletal progenitor cells. Acta Biomater. 2023;166:109-118. doi: 10.1016/j.actbio.2023.05.039.
 Medicine Innovates Medicine Innovates: Delivering innovations in medicine to the world for better health and prosperity
Medicine Innovates Medicine Innovates: Delivering innovations in medicine to the world for better health and prosperity
