Significance
Multiple Sclerosis (MS) is a chronic inflammatory, demyelinating, and neurodegenerative disorder of the central nervous system (CNS) that affects the white and grey matter of the brain, spinal cord, and optic nerve. MS is one of the most common causes of non-traumatic disability among young and middle-aged adults. MS is characterized by impaired remyelination, axonal degeneration, and progressive loss of motor function. However, current animal models do not adequately assess whether drugs for remyelination lead to functional recovery.
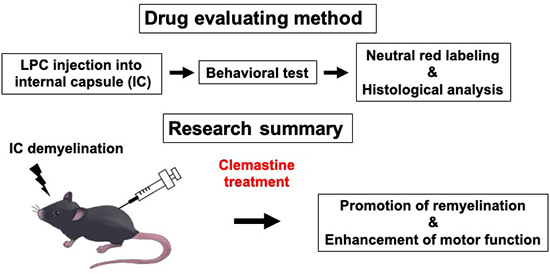
In a recent study published in the peer-reviewed Journal Neurochemistry International, Assistant Professor Reiji Yamazaki, Assistant Professor Yasuyuki Osanai, Dr. Tom Kouki, and Professor Nobuhiko Ohno from Jichi Medical University, in collaboration with Associate Professor Jeffrey K. Huang from Georgetown University, introduced a new mouse model of lysophosphatidylcholine (LPC)-induced internal capsule (IC) demyelination. The novel model presents exciting possibilities for assessing drug effects on remyelination and motor function. Their research focused on investigating the effects of clemastine, an anti-muscarinic drug known to promote oligodendrocyte differentiation and remyelination, in the new IC demyelination mouse model.
The authors chose IC demyelination model as it closely mimics the corticospinal tract damage observed in MS and white matter stroke patients, leading to motor dysfunction. The researchers successfully confirmed the demyelination site through various methods, including neutral red (NR) labeling and immunofluorescence staining. To assess motor deficits, grip strength and wire hanging tests were employed, showing significantly decreased grip strength in demyelinated mice. These results established that the grip strength test is a reliable indicator of motor impairment due to IC damage.
The research team injected clemastine in mice with IC demyelination and demonstrated promising outcomes. Clemastine treatment improved motor function and ameliorated asymmetric motor paralysis. Additionally, it increased oligodendrocyte density and significantly enhanced remyelination, suggesting a positive relationship between clemastine and functional recovery in this mouse model.
IC demyelination induces asymmetric motor deficits in mice, leading to changes in forepaw preference. The authors used the cylinder test to assess forepaw use between PBS- and clemastine-treated mice. The percentage of right forepaw usage decreased significantly in clemastine-treated mice, while the percentage of left forepaw usage remained unaffected. Furthermore, axonal dystrophy is known to contribute to motor deficits after demyelination. Through immunofluorescence staining, the researchers detected axonal swellings in both PBS- and clemastine-treated mice. However, SMI32/NF-double positive signals, indicative of axonal injury, showed a trend of decrease in clemastine-treated mice. This suggests that clemastine may reduce axonal injury and contribute to functional recovery.
When the authors conducted electron microscopy (EM) analysis, results showed the effect of clemastine on remyelination. Clemastine treatment from 3 to 12 days post lesion (dpl) positively influenced the remyelination process, promoting the differentiation of oligodendrocytes that produce myelin in the demyelinated areas.
According to the authors, the new IC demyelination mouse model, combined with NR labeling for EM analysis, represents a valuable tool for MS research. It offers the ability to not only study histological changes but also evaluate motor functional recovery after drug treatment. The study’s findings support previous reports that clemastine has potential therapeutic value for MS and other demyelinating diseases. Moreover, the model provides a means to assess the effects of different drug treatments on remyelination-mediated motor function.
In conclusion, the research team reported a novel mouse model of IC demyelination, which will expedite pre-clinical studies on remyelination and motor function. The research reveals the significant potential of clemastine in promoting remyelination and enhancing motor function recovery. Moreover, the IC demyelination mouse model, combined with NR labeling for EM analysis, opens new avenues for MS research and drug development. The ultimate goal is to translate these findings into tangible benefits for patients, improving their quality of life and providing hope for those affected by MS and related conditions.
Reference
Yamazaki R, Osanai Y, Kouki T, Huang JK, Ohno N. Pharmacological treatment promoting remyelination enhances motor function after internal capsule demyelination in mice. Neurochem Int. 2023;164:105505. doi: 10.1016/j.neuint.2023.105505.
 Medicine Innovates Medicine Innovates: Delivering innovations in medicine to the world for better health and prosperity
Medicine Innovates Medicine Innovates: Delivering innovations in medicine to the world for better health and prosperity

