Significance
Alzheimer’s disease (AD) is a devastating neurodegenerative condition characterized by profound cognitive decline and memory loss. The disease presents an immense challenge due to the lack of efficient diagnostic tools and effective treatment strategies. One of the hallmark pathological features of AD is the accumulation of senile plaques primarily composed of amyloid-β (Aβ) aggregates. These plaques manifest in the early stages of the disease, often many years before any symptomatic deficiencies or clinical diagnosis occur. The recognition of Aβ aggregation in the brain of AD patients has become a significant diagnostic criterion, highlighting the critical need for the development of potent probes to monitor Aβ-related processes, including aggregation and fibrillization. Over the years researchers have successfully developed various types of probes for in vivo and in vitro Aβ detection for early diagnosis of AD. These probes can be categorized into five main groups: magnetic resonance imaging, positron emission tomography (PET), single photon emission computed tomography, near-infrared (NIR) fluorescence probes, and photoacoustic imaging probes. Among these, PET probes have been the primary diagnostic tools for detecting cerebral Aβ in AD patients. However, their clinical use is limited due to issues such as radiation exposure and high costs, as well as a lack of specificity. Therefore, there has been a growing interest in the development of NIR fluorescence probes for in vitro and in vivo Aβ imaging. Recently a number of NIR fluorescence imaging probes have emerged, aimed at detecting Aβ plaques in vitro and in vivo. Pioneering work in this field began with the discovery of various NIR fluorescence probes, such as AOI987 and hydrophobic planarizable bithiophene-based probes. Subsequently, numerous probes with diverse chemical and electronic structures, including those containing dicyanomethylene acceptors, boron dipyrromethane (BODIPY), or difluoroboronate-coupled curcumin scaffolds, have been developed. These probes have shown promise in recognizing Aβ plaques, yet there remains a pressing need for novel NIR fluorescence probes with enhanced binding affinity and improved in vivo imaging capabilities.
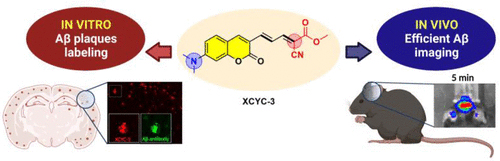
In a new study published in the ACS Chemical Neuroscience Journal, led by Professor Ming-Hua Xu and Professor Haiyan Zhang, in collaboration with researchers from the Shenzhen Grubbs Institute, the Department of Chemistry at the Southern University of Science and Technology, and the Shanghai Institute of Materia Medica, Chinese Academy of Sciences developed a novel coumarin-derived small molecule fluorophore known as XCYC-3. This innovative probe boasts a simple structure and has exhibited high fluorescent enhancement for Aβ aggregates, making it a compelling candidate for in vitro and in vivo Aβ plaque detection. Notably, the in vivo experiments involving XCYC-3 have demonstrated its potential as a promising fluorescence probe for the recognition of cerebral Aβ plaques in animal models.
The development of XCYC-3 is underpinned by the rationale that push−pull fluorophores, with terminal donor and acceptor groups connected by a π-conjugated bridge, have shown significant promise in detecting Aβ aggregates. The authors have previously designed probes with extended π-conjugation, resulting in efficient in vivo detection of Aβ plaques. Building on this knowledge, the team in the new study decided to investigate whether the incorporation of a coumarin framework into the π-bridge between donor and acceptor groups would yield specific fluorescent probes for Aβ detection. To construct XCYC-3, the authors combined the coumarin moiety with conjugated double bonds, maintaining a dimethylamino group as the electron donor and a dicyanomethylene group as the acceptor. By varying the length of conjugated double bonds and introducing different substituents in the acceptor moiety, XCYC-3 emerged as a promising candidate. The synthesis of XCYC-3 and related compounds involved a well-established procedure, resulting in easily accessible probe molecules. The evaluation of XCYC-1, XCYC-2, and XCYC-3 revealed intriguing photophysical properties. As the conjugation of double bonds increased (in XCYC-2 and XCYC-3), the emission wavelength showed a pronounced redshift, reaching 641 and 632 nm, respectively. The fluorescence quantum yield in dimethyl sulfoxide (DMSO) also improved significantly when a cyano group was replaced with a methyl acetate group in XCYC-3. Moreover, these compounds exhibited optimal log P values for their intended application. Afterward, the authors conducted in vitro fluorescent staining of Aβ plaques in brain sections of 5xFAD mice, a well-known AD transgenic mouse model, which provided valuable insights into the imaging capabilities of XCYC-1, XCYC-2, and XCYC-3. While XCYC-1 showed low fluorescence signals, XCYC-2, with an extended π-conjugation, displayed improved staining. Most notably, XCYC-3 exhibited substantially stronger fluorescence signals compared to the other two compounds, indicating its superior imaging ability for Aβ deposits. XCYC-3’s effectiveness was further confirmed through histological co-staining experiments, revealing its selective binding to Aβ plaques.
The researchers conducted comprehensive investigation on the fluorescence responses of XCYC-3 to Aβ monomers and Aβ1−42 aggregates. They observed concentration-dependent fluorescent enhancements when Aβ monomers were incubated with XCYC-3. However, the coincubation of XCYC-3 with Aβ1−42 aggregates resulted in even greater fluorescence enhancement compared to Aβ monomers, highlighting the probe’s stronger interaction with Aβ aggregates. Crucially, XCYC-3 exhibited no response to various amino acids, confirming its specificity. The binding affinity of XCYC-3 to Aβ aggregates was quantified, with a Kd of 71.11 nM, underscoring its potent binding. Moreover, when the authors performed molecular docking simulations to discover the interaction mechanism between XCYC-3 and Aβ aggregates. The simulations revealed that XCYC-3 could form hydrogen bonds with specific amino acids within Aβ aggregates, indicating a high level of specificity. These docking studies reinforced the notion that XCYC-3 selectively binds to Aβ aggregates, making it a valuable tool for Aβ detection. One of the most exciting aspects of this research was the in vivo imaging feasibility of XCYC-3 for Aβ plaques in AD transgenic mice. The study confirmed that XCYC-3 had good blood-brain barrier permeability and that its central fluorescent signals primarily derived from XCYC-3, rather than its demethylated form. Importantly, in vivo imaging experiments demonstrated that XCYC-3 efficiently recognized Aβ plaques in the brains of AD transgenic mice. These findings were further validated through ex vivo measurements on brain slices, which revealed clear fluorescent spots that were well colocalized with Aβ antibody signals.
In a nutshell, the discovery of XCYC-3, a coumarin-derived small molecule fluorophore, offers a promising probe for the early detection and monitoring of Aβ plaques in AD. Its superior binding affinity to Aβ aggregates, combined with its ability to penetrate the blood-brain barrier, positions XCYC-3 as a valuable tool for both in vitro and in vivo imaging. These findings open the door to the development of more potent and specific near-infrared fluorescence probes for the diagnosis and management of Alzheimer’s disease.
Reference
Cao Y, Liu X, Zhang J, Liu Z, Fu Y, Zhang D, Zheng M, Zhang H, Xu MH. Design of a Coumarin-Based Fluorescent Probe for Efficient In Vivo Imaging of Amyloid-β Plaques. ACS Chem Neurosci. 2023;14(5):829-838. doi: 10.1021/acschemneuro.2c00468.
 Medicine Innovates Medicine Innovates: Delivering innovations in medicine to the world for better health and prosperity
Medicine Innovates Medicine Innovates: Delivering innovations in medicine to the world for better health and prosperity
