Significance
Lipids constitute a significant portion of the brain’s dry mass, playing crucial roles in cellular structure, signaling, and energy storage. Astrocytes, pivotal in brain lipid metabolism, engage in lipid exchange, synthesis, and detoxification. The maintenance of lipid homeostasis is intricate, involving various cellular components including lipid droplets (LDs), which store neutral lipids and serve as energy reserves. Dysregulation of lipid metabolism has been implicated in neurodegenerative diseases, including Alzheimer’s Disease (AD), where lipid accumulation has been observed. Apolipoprotein E (APOE), primarily produced by astrocytes and microglia, is a key player in lipid transport and metabolism in the brain, with its E4 variant being a significant genetic risk factor for AD. However, the exact mechanisms by which APOE influences lipid metabolism and contributes to AD risk remain to be fully elucidated.
A new paper published in Journal of Cell Biology by Ian Windham, Alex Powers, Joey Ragusa, E Diane Wallace, Maria Clara Zanellati, Victoria Williams, Colby Wagner, Kristen White and Sarah Cohen from the Department of Cell Biology and Physiology, University of North Carolina at Chapel Hill, the authors conducted an investigation to understand the role of APOE in astrocyte lipid metabolism, focusing on its interaction with LDs and the implications for AD, particularly in the context of the APOE4 variant. The study utilized immortalized astrocyte cell lines expressing human APOE3 or APOE4, primary rat cortical astrocytes, and a range of molecular biology, biochemistry, and imaging techniques.
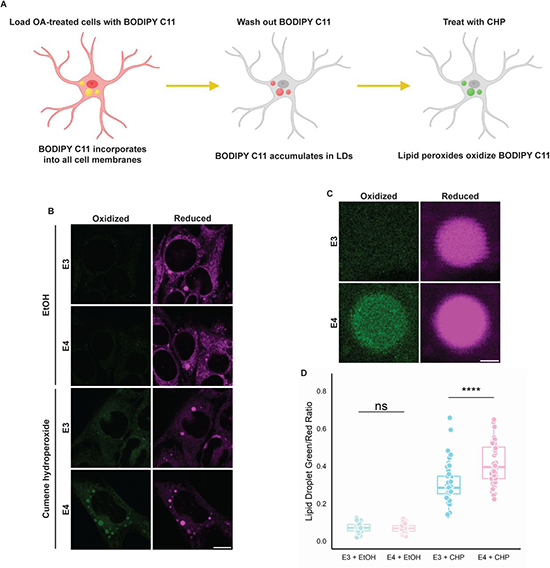
The researchers demonstrated that APOE can localize to the cytoplasmic surface of LDs in astrocytes, diverging from its traditional route to secretion. This redirection of APOE to LDs was observed under conditions promoting neutral lipid synthesis, implicating APOE in the regulation of LD biogenesis and turnover. APOE knockdown resulted in the formation of fewer but larger LDs, enriched in unsaturated triglycerides. Remarkably, this phenotype could be rescued by expressing a chimeric APOE that exclusively targets LDs. Similarly, astrocytes expressing APOE4 formed larger LDs with compromised turnover and heightened sensitivity to lipid peroxidation, suggesting a deleterious gain of function associated with the APOE4 variant. The study provided insights into the trafficking of APOE to LDs, involving its movement from the cytoplasmic face of the endoplasmic reticulum (ER) to LDs through membrane bridges, bypassing traditional secretion pathways. This novel pathway underscores the complexity of intracellular lipid trafficking and APOE’s multifaceted role in lipid metabolism. The authors highlighted that LD-associated APOE plays a critical role in regulating LD size and composition. APOE on LDs appears to influence the distribution and saturation levels of triglycerides within LDs, thereby impacting cellular lipid homeostasis and potentially the cell’s response to stress.
The findings suggest that the APOE4 variant’s influence on LD morphology and composition could be a contributing factor to AD risk. Larger, unsaturated LDs in APOE4-expressing astrocytes, with their impaired turnover and increased peroxidation sensitivity, may exacerbate lipid dysregulation and inflammation, fueling AD pathology. Moreover, because AD is a complex neurodegenerative disorder with multifaceted pathogenesis, including genetic, environmental, and metabolic factors. APOE4 is the most significant genetic risk factor for late-onset AD, yet the mechanisms through which it contributes to disease risk are not fully understood. This research sheds light on a novel aspect of APOE4’s role in lipid metabolism dysregulation, offering insights into how genetic predispositions can influence disease progression. Moreover, the brain is lipid-rich, and proper lipid metabolism is crucial for its function. Dysregulation of lipid metabolism has been implicated in various neurodegenerative diseases, including AD. By demonstrating how APOE and its variant APOE4 interact with LDs in astrocytes, this study connects lipid metabolic processes directly to neurodegenerative disease mechanisms, providing a clearer picture of how metabolic disturbances can contribute to neuronal damage and dysfunction. Furthermore, understanding the specific role of APOE in LD dynamics opens up potential new therapeutic avenues. Modulating APOE’s interaction with LDs, particularly in the context of the APOE4 variant, could offer new strategies for managing or preventing AD. Targeting the lipid metabolism pathways influenced by APOE might mitigate some of the pathological features associated with AD, such as lipid peroxidation and inflammation.
The new study by Professor Cohen illuminates a previously unrecognized facet of APOE’s function in astrocyte lipid metabolism, linking it directly to the regulation of LD dynamics. The aberrant behavior of APOE4 in this context offers a new perspective on how this variant may predispose individuals to AD, highlighting the interplay between lipid metabolism and neurodegenerative disease pathology. These findings open up new avenues for exploring therapeutic strategies targeting lipid metabolism in AD, particularly in modulating APOE’s interaction with LDs. Further research is needed to unravel the precise molecular mechanisms underlying APOE’s targeting to LDs and its functional implications in AD and other lipid-associated disorders.
Reference
Windham IA, Powers AE, Ragusa JV, Wallace ED, Zanellati MC, Williams VH, Wagner CH, White KK, Cohen S. APOE traffics to astrocyte lipid droplets and modulates triglyceride saturation and droplet size. J Cell Biol. 2024 Apr 1;223(4):e202305003. doi: 10.1083/jcb.202305003.
 Medicine Innovates Medicine Innovates: Delivering innovations in medicine to the world for better health and prosperity
Medicine Innovates Medicine Innovates: Delivering innovations in medicine to the world for better health and prosperity

