Significance
Pelvic Organ Prolapse (POP) is a common condition affecting a significant proportion of women, particularly those who have experienced vaginal childbirth. In POP, the organs in the pelvis, such as the bladder, uterus, and rectum, drop from their normal position and bulge into the vagina, and may even bulge outside the body. This can happen when the tissues supporting these organs become weak. The traditional management of POP involves either surgical intervention or the use of pessaries, which are devices inserted into the vagina to support and elevate prolapsed organs. However, traditional pessaries are reusable only and have several drawbacks, including discomfort, the need for frequent medical supervision for insertion and removal, and the risk of adverse events such as infections and vaginal wall trauma. A new study published in Frontiers in Medicine by investigators Dr. Elan Ziv and Mrs. Tsvia Erlich from ConTIPI Medical Ltd., who developed and tested a novel, self-inserted, disposable vaginal prolapse device named ProVate, aimed at the non-surgical management of POP. Their goal was to offer a more user-friendly and comfortable alternative to traditional ring pessaries, addressing common issues such as discomfort, difficulty in insertion and removal, usage of a reusable only device, and the need for frequent medical supervision.
The authors’ study was a randomized, controlled, statistically powered, multicenter trial conducted at seven outpatient clinics. It was designed as an open-label, cross-over, non-inferiority trial, focusing on home use of ProVate compared to a traditional ring pessary. The researchers enrolled 85 women with symptomatic POP, out of which 71 were randomized to either start with ProVate or a control (currently available ring pessary), and 58 completed the study per protocol. Participants used each device for a specified period, followed by a washout phase before switching to the other device. The primary outcomes assessed were the safety aspects of the device. In addition, objective reduction of POP, measured by the Pelvic Organ Prolapse Quantification System (POP-Q) was evaluated. Safety was assessed by the incidence and severity of adverse events. An additional safety aspect evaluated in the study was the device’s impact on vaginal microflora (published separately).
The authors showed that ProVate has high effectiveness in reducing POP, with 90.7% of users experiencing complete prolapse reduction to stage 0 (no prolapse), and 96.3% achieving reduction to stage 0 or 1 (no prolapse). These results were comparable to the control group, where 82.5% experienced complete prolapse reduction, and 91.2% had reductions to stage 0 or 1. The incidence of adverse events was similar between the ProVate and control groups, with most adverse events being minor, mild, and anticipated. Notably, ProVate did not significantly alter vaginal microflora compared to the control, indicating its safety for prolonged use. Moreover, ProVate’s design as a self-insertable device and user friendly with an applicator and its disposability were highlighted as major advantages, potentially improving compliance, and substantially reducing the need for medical visits for device management beyond routine follow up.
The study also highlighted the user-friendly design of ProVate, which is a significant advantage over traditional pessaries. ProVate comes ready for use, within a single wrapper, as with the packaging of a regular menstrual tampon, in a slender shape and small dimensions, within an applicator. ProVate is a self-insertable only device, intended for home use, by the user herself, regardless of time and place, and without the need for medical supervision for insertion and removal. Following insertion, the device and the applicator separate, and the applicator is discarded. The device may remain in the vagina for up to seven days, as the user desires, and a pull on a string collapses the device into small dimensions and easy removal for disposal. This self-controlled nature of the device could significantly improve the quality of life for women with POP. Figure 1 shows the ProVate device in its different configurations, from insertion to removal.
This ease of use is expected to reduce the rate of discontinuation, a common issue with traditional pessaries due to difficulties in insertion, removal, and the dependency upon the clinic with regular medical appointments for cleaning and re-insertion, and non-regular visits for adverse events. Moreover, the disposable aspect of ProVate addresses concerns related to hygiene and the risk of infections associated with the long-term use of non-disposable pessaries. The ability to replace the device frequently without medical intervention could lead to better adherence to treatment and potentially lower the risk of adverse events. In conclusion, the study by Ziv and Erlich provides valuable evidence on the efficacy and safety of ProVate as a novel approach to the non-surgical management of POP. The findings suggest that ProVate could be a viable alternative to traditional ring pessaries, offering similar efficacy with the added benefits of ease of use, disposability, and potentially improved compliance. Further research is warranted to explore the long-term outcomes of ProVate use, including its impact on quality of life, sexual function, and cost-effectiveness compared to traditional pessaries and surgical options.
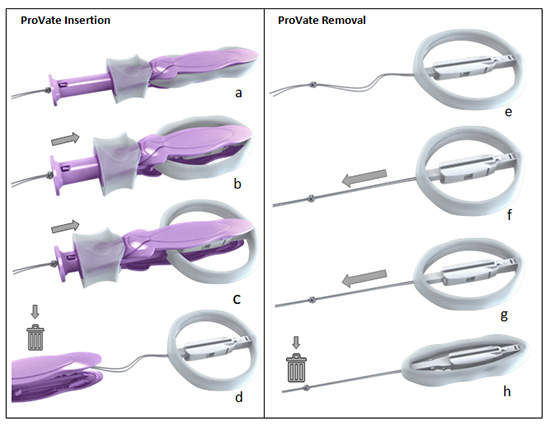
The ProVate device is provided clean, within a personal wrap, readily available for immediate vaginal insertion, in small dimensions, within a disposable applicator (a). Following vaginal insertion, resembling the concept of insertion of the menstrual tampon, the plunger is pushed and the slender compacted device within the applicator gradually enlarges to become a ring (b). By the end of pushing of the plunger, the ring becomes fully deployed (c). The applicator then separates from the ring, and is removed from the vagina for disposal, leaving the string available for later removal (d). The deployed ring may remain in the vagina for up to 7 days (e). A pull on the string collapses the ring into is its slender pre-insertion size, for comfortable removal and disposal (f-h).
Reference
Ziv E, Erlich T. A randomized controlled study comparing the objective efficacy and safety of a novel self-inserted disposable vaginal prolapse device and existing ring pessaries. Front Med (Lausanne). 2023;10:1252612. doi: 10.3389/fmed.2023.1252612.
 Medicine Innovates Medicine Innovates: Delivering innovations in medicine to the world for better health and prosperity
Medicine Innovates Medicine Innovates: Delivering innovations in medicine to the world for better health and prosperity
