Significance
Antimicrobial resistance (AMR) represents one of the most pressing public health concerns of the 21st century, threatening to render many of our most potent antimicrobial agents ineffective. The World Health Organization has identified AMR as a critical area requiring urgent action, with fluoroquinolones, a class of broad-spectrum antibiotics that target bacterial DNA gyrase and topoisomerase IV, listed among the “highest priority critically important antimicrobials.” However, the rise of target-mediated resistance has significantly undermined the clinical utility of fluoroquinolones, necessitating the development of novel antibacterial agents.
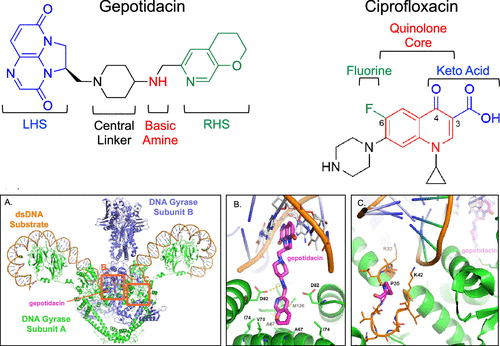
A new study published in ACS Infectious Diseases and conducted by Dr. Alexandria Oviatt, Dr. Elizabeth Gibson, Dr. Jianzhong Huang, Dr. Karen Mattern, Dr. Keir Neuman, Dr. Pan Chan, and Professor Neil Osheroff from the Vanderbilt University School of Medicine, the authors investigated the efficacy of gepotidacin, a novel triazaacenaphthylene antibacterial, against Escherichia coli (E. coli), focusing on its interaction with bacterial DNA gyrase and topoisomerase IV, critical enzymes in bacterial DNA replication and maintenance. Gepotidacin, a first-in-class triazaacenaphthylene antibacterial, emerges as a promising candidate in this endeavor. Its unique mechanism of action, targeting both DNA gyrase and topoisomerase IV in Escherichia coli, sets it apart from fluoroquinolones and offers a new avenue for antibiotic development. The ability of gepotidacin to induce single-stranded DNA breaks, unlike the double-stranded breaks associated with fluoroquinolones, highlights a potentially less genotoxic profile and a different therapeutic pathway for bacterial eradication.
The researchers used purified enzymes and performed in vitro supercoiling and decatenation assays. These assays measured gepotidacin’s ability to inhibit the activity of gyrase (which introduces negative supercoils into DNA) and topoisomerase IV (which decatenates intertwined DNA circles), respectively. They found that Gepotidacin displayed potent inhibition of both gyrase and topoisomerase IV at sub-micromolar concentrations, suggesting well-balanced dual targeting. This balanced activity is crucial for minimizing the development of resistance since mutations would need to occur in both enzyme targets to significantly impact bacterial susceptibility.
To explore the nature of DNA breaks induced by gepotidacin and compare them to those induced by fluoroquinolones, which typically cause double-stranded breaks, the team conducted DNA cleavage assays using both gyrase and topoisomerase IV in the presence of gepotidacin and analyzed the DNA products through gel electrophoresis to distinguish between single-stranded and double-stranded DNA breaks. They found that Gepotidacin preferentially induced single-stranded DNA breaks in its interaction with both enzymes. Unlike fluoroquinolones, which stabilize double-stranded DNA breaks leading to lethal damage, gepotidacin’s induction of single-stranded breaks suggests a different, potentially less cytotoxic mechanism of action. Moreover, the researchers introduced specific mutations into the genes encoding gyrase and topoisomerase IV. These mutations were chosen based on their potential to interfere with gepotidacin binding. They then assessed the impact of these mutations on bacterial susceptibility to gepotidacin through MIC (minimum inhibitory concentration) assays. According to the authors, mutations in specific amino acids involved in gepotidacin’s interaction with gyrase and topoisomerase IV altered the compound’s efficacy against the enzymes and, consequently, against E. coli. Notably, mutations had to be present in both targets to significantly reduce the antibacterial activity of gepotidacin, underscoring its dual-targeting strategy as a mechanism to deter resistance development. Furthermore, to evaluate the clinical efficacy of gepotidacin in treating uncomplicated urinary tract infections (UTIs) caused by E. coli, the research team conducted phase III clinical trials, comparing gepotidacin’s effectiveness to that of nitrofurantoin, a standard treatment for UTIs. Gepotidacin demonstrated noninferiority to nitrofurantoin in treating uncomplicated UTIs and showed statistically significant superiority in some aspects. These results support gepotidacin’s potential as an effective treatment option for UTIs caused by E. coli.
Indeed, the demonstrated efficacy of gepotidacin in phase III clinical trials for the treatment of uncomplicated urinary tract infections, and its noninferiority to existing treatments, underscores its potential as a valuable addition to our antimicrobial arsenal. However, the broader implications of gepotidacin’s mechanism of action extend beyond its immediate clinical applications. By providing a blueprint for the development of antibiotics with dual-targeting capabilities and reduced potential for resistance development, gepotidacin represents a significant step forward in our ongoing battle against AMR. The dual-targeting mechanism of gepotidacin against gyrase and topoisomerase IV in E. coli is particularly noteworthy. This balanced inhibition is crucial for minimizing the development of resistance, as mutations in both targets would be required for significant resistance emergence. This study’s exploration of mutations affecting gepotidacin’s activity further elucidates the mechanisms underlying potential resistance and highlights the importance of targeting multiple sites to combat AMR effectively.
Overall, the detailed examination of gepotidacin’s interaction with bacterial DNA gyrase and topoisomerase IV revealed its potent dual-targeting mechanism, ability to induce single-stranded DNA breaks, and a strategic approach to minimizing resistance development. These findings, combined with its demonstrated clinical efficacy, position gepotidacin as a promising new antibacterial agent against E. coli infections, with potential applications in treating a broader range of bacterial diseases.
Reference
Alexandria A. Oviatt, Elizabeth G. Gibson, Jianzhong Huang, Karen Mattern, Keir C. Neuman, Pan F. Chan*, and Neil Osheroff*. Interactions between Gepotidacin and Escherichia coli Gyrase and Topoisomerase IV: Genetic and Biochemical Evidence for Well-Balanced Dual-Targeting. ACS Infect. Dis. 2024 https://pubs.acs.org/doi/10.1021/acsinfecdis.3c00346
 Medicine Innovates Medicine Innovates: Delivering innovations in medicine to the world for better health and prosperity
Medicine Innovates Medicine Innovates: Delivering innovations in medicine to the world for better health and prosperity


