Significance
Monoclonal antibodies (mAbs) have revolutionized medical therapies and diagnostics. Their high specificity and efficacy have made them invaluable in treating many diseases, from cancer to autoimmune disorders. The global demand for these biologics has been increasing steadily, leading to intense competition among drug manufacturers. However, the purification of mAbs for industrial use is a complex and challenging process, primarily due to the high purity and quality standards required for therapeutic applications and the purification process must effectively remove various impurities such as host cell proteins, DNA, viruses, and other impurities. Another factor to consider during the purification process is maintaining the stability and integrity of mAbs and pH, temperature, and ionic strength need to be carefully controlled to prevent denaturation or aggregation of the antibodies.
Recently, advancements in cell lines and media and bioreactor conditions optimization have led to high cell densities and correspondingly high antibody titers. However, this success has brought its own set of challenges. High antibody concentrations have made purification processes more complex and costly, accounting for a significant portion of production costs. Downstream processing, particularly the purification of mAbs, has become a major hurdle. Traditional methods, like continuous centrifugation and depth microfiltration, are less effective at higher antibody concentrations. Additionally, chromatographic steps, especially those using Protein A affinity chromatography, are expensive and have their limitations, including the risk of antibody aggregation under acidic conditions.
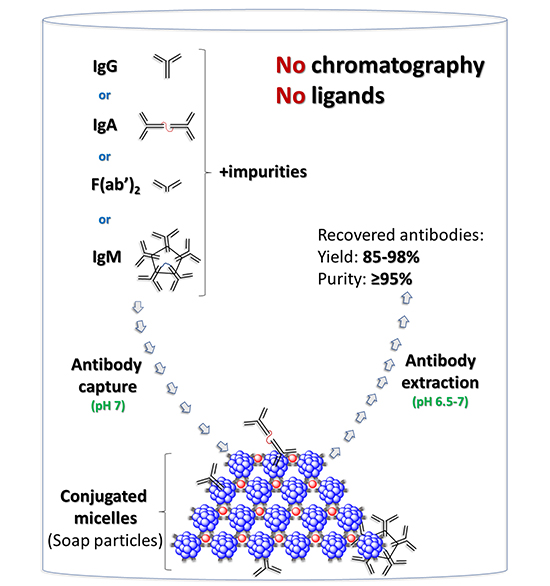
To address these limitations, a new study published in the Journal Nano Select by Dr. Gunasekaran Dhandapani, Dr. Ellen Wachtel, and Dr. Guy Patchornik from the Ariel University in Israel, the authors introduced a novel non-chromatographic method for purifying human Immunoglobulin G (hIgG) from cell cultures, especially at high concentrations. The process operates at near-neutral pH levels, avoiding the potential denaturation and aggregation of antibodies. This approach has demonstrated high yields (84-99%) and maintained the monomeric state of antibodies, as confirmed by dynamic light scattering (DLS) analysis.
The researchers designed a non-chromatographic method using conjugated, mixed-micelle aggregates. These aggregates comprised non-ionic detergents, tyrosine monomers, and an amphiphilic [(bathophenanthroline)3:Fe2+] complex. The goal was to create a system that could efficiently purify hIgG without the need for traditional chromatographic techniques, which can be costly and less effective at higher antibody concentrations.
The team prepared mixed-micellar aggregates using the selected detergents, tyrosine, and the amphiphilic chelator. Then they captured hIgG from E. coli lysates using these aggregates. Afterward, the captured hIgG was then extracted from the aggregates under near-neutral pH conditions, which was crucial to avoid denaturation and aggregation of the antibodies. The researchers tested the scalability of their method by increasing the process volume from 0.1 to 5 mL. They demonstrated that the new process could be scaled up without loss of yield or purity. The authors also employed several analytical techniques to assess the efficacy of the purification process: for instance, they used SDS-PAGE (Sodium Dodecyl Sulphate Polyacrylamide Gel Electrophoresis) to determine the purity of the recovered hIgG. They also employed DLS to assess the size distribution of the purified hIgG and confirm its monomeric state. Additionally, the researchers conducted spike-and-recovery experiments with hIgG at different concentrations in the presence of E. coli lysate. They measured the yield and purity of the recovered hIgG to quantify the effectiveness of their purification process.
According to the authors, there is significant advantages of the new method; for instance, it achieved almost quantitative recovery of hIgG with high purity, even at concentrations of 15-25 mg mL−1. Moreover, upscaling the process from 0.1 to 5 mL required only proportional increases in all reagents, without affecting the yield or antibody purity. By operating at pH 6.5-7, the method avoided exposing antibodies to acidic conditions, thereby minimizing the risk of denaturation and aggregation. Furthermore, unlike chromatographic methods, this approach does not rely on expensive ligands, potentially reducing costs.
The novel purification method reported by Dr. Guy Patchornik and colleagues has significant implications for the pharmaceutical industry. It offers a cost-effective and efficient alternative to traditional chromatographic purification methods, particularly for high-concentration antibody solutions. The scalability and adaptability of this approach make it a promising candidate for industrial applications. While the study presents a promising alternative to existing purification methods, further research is needed to fully understand its implications in industrial settings. Issues such as handling larger volumes typical of industrial processes, dealing with mammalian cell content as a contamination background, and integrating this method with other steps in the purification process need to be addressed. As the demand for monoclonal antibodies continues to grow, such innovations are crucial in ensuring the efficient and cost-effective production of these vital therapeutic agents.
References
Gunasekaran Dhandapani, EllenWachtel, Guy Patchornik. Conjugated surfactant micelles: A non-denaturing purification platform for concentrated human immunoglobulin G. Nano Select 2023;4:386-394.
Gunasekaran Dhandapani, Ellen Wachtel, Ishita Das, Mordechai Sheves, Guy Patchornik. Conjugated detergent micelles as a platform for IgM purification. Biotechnology and Bioengineering 2022;119:1997-2003.
Go To Biotechnology and Bioengineering
Gunasekaran Dhandapani, Ellen Wachtel, Ishita Das, Mordechai Sheves, Guy Patchornik. Purification of antibody fragments via interaction with detergent micellar aggregates. Scientific Reports 2021; 11(1):11697-11708.
 Medicine Innovates Medicine Innovates: Delivering innovations in medicine to the world for better health and prosperity
Medicine Innovates Medicine Innovates: Delivering innovations in medicine to the world for better health and prosperity
