Significance
The human gastrointestinal system is a marvel of complexity, orchestrating a symphony of events to process ingested food and extract essential nutrients. At the heart of this digestive symphony lies the stomach, a dynamic organ that not only digests food but also ensures its timely passage into the small intestine for absorption. Central to this process are the rhythmic contractions of the gastric antrum smooth muscle, which mix ingested food with digestive enzymes and stomach acid, propelling the resulting chyme into the duodenum for further processing and absorption. These contractions must be finely tuned to match the pace of digestion, ensuring optimal nutrient absorption while avoiding complications such as gastric emptying disorders.
Milk Fat Globule Epidermal Growth Factor 8 (Mfge8), also known as lactadherin or Secreted Epidermal Growth Factor Repeat and Discoidin Domain-Containing Protein 1 (SED-1), was originally identified in mammary epithelial cells, where it was associated with milk fat globules. Over time, research has unveiled a much broader role for Mfge8 in various cells and tissues throughout the body. Notably, Mfge8 participates in diverse physiological responses involving cell-cell interactions and even plays a role in blood coagulation and the clearance of phosphatidylserine-expressing red blood cells.
In a new study published in the peer-reviewed American Journal of Physiology-Cell Physiology by Professor Brian Perrino, Justin Malogan, and Caroline Cobine from the Department of Physiology and Cell Biology at University of Nevada Reno School of Medicine in collaboration with Dr. Kent Sasse from the Nevada Surgical Associates conducted a comprehensive study aimed at understanding the role of Mfge8 in the regulation of gastric antrum smooth muscle contractions and gastric emptying. They employed a combination of molecular biology techniques, histological analysis, and immunofluorescence staining to investigate for the first time the expression and distribution of Mfge8 within the human gastric antrum.
Perhaps the most significant finding of this research is the identification of Mfge8-expressing perivascular cells, specifically those marked by the transcription factor Mef2c. These cells were predominantly located in the submucosa of the human gastric antrum. This revelation raises intriguing questions about the role of Mfge8 in the gastrointestinal tract and its implications for digestive health. These Mef2c-positive perivascular cells, located in the submucosa, appear to be a novel subtype of pericytes. Pericytes, known for their critical roles in regulating vascular network angiogenesis, morphogenesis, and function, have now been implicated in the regulation of gastric antrum smooth muscle contractility.
Approximately 80% of Mfge8-expressing cells were found to be Mef2c-positive, emphasizing the strong association between Mfge8 and these perivascular cells. This discovery challenges our previous understanding of Mfge8’s distribution within the body and prompts further investigation into the functions of Mfge8-expressing perivascular cells.
To gain deeper insights into the potential functions of Mfge8-expressing perivascular cells, the authors examined their coexpression patterns with various markers. Notably, Mfge8 was found to be coexpressed with NG2 and Platelet-Derived Growth Factor Receptor B, suggesting that these perivascular Mef2c-positive cells are pericytes. Pericytes, traditionally associated with vascular support, are now implicated in the regulation of gastric antrum motility and gastric emptying, marking a significant shift in our understanding of their roles. Interestingly, the coexpression patterns revealed a mosaic of cellular phenotypes. Cells coexpressing Mfge8 and Mef2c, Mfge8 and NG2, and Mfge8 and ACTA2 (alpha-smooth muscle actin) hinted at the multifaceted nature of these perivascular cells. Importantly, Mfge8 was not found to be expressed in the smooth muscle cells marked by ACTA2 or the endothelial cells marked by CD34, excluding these cell types from its primary expression profile. This specificity highlights the unique association of Mfge8 with perivascular cells.
According to the authors, there is a potential protective role of Mfge8-expressing perivascular cells in maintaining the integrity of capillary beds. The gastric antrum’s vigorous contractions expose the mucosal and muscular microvasculature to external forces, which could increase the risk of damage or obstruction. Mfge8’s involvement in modulating these contractions suggests that it may serve as a defense mechanism to safeguard capillary beds from disruption. This newfound understanding of Mfge8’s role in protecting the microcirculation adds another layer of complexity to our comprehension of gastrointestinal physiology. It highlights the sophisticated mechanisms at play in ensuring not only efficient digestion but also the preservation of the delicate vascular network that supports the GI system’s diverse functions.
The discovery of Mfge8’s involvement in gastric antrum smooth muscle regulation opens doors to potential therapeutic applications. Gastrointestinal disorders, including those related to gastric motility and gastric emptying, can profoundly impact an individual’s quality of life. Understanding the role of Mfge8 in modulating these contractions could pave the way for innovative interventions aimed at restoring or optimizing gastric motility in cases of dysregulation. By reducing the contractile activity of the gastric antrum, Mfge8 slows down the gastric emptying rate. The weight loss drugs Ozempic, Trulicity, and Wegovy belong to a class of medications called glucagon-like peptide-1 (GLP-1) receptor agonists. GLP-1 is a naturally occurring hormone in the body that promotes weight loss by several mechanisms, including slowing down gastric emptying. This contributes to feelings of fullness and satiety, resulting in reduced food consumption and, ultimately, weight loss. These findings raise the intriguing possibility that a drug that mimics the inhibitory actions of Mfge8 on gastric emptying might also be effective for promoting weight loss.
As we reflect on this novel discovery, several questions and avenues for future research come to the forefront: What are the specific mechanisms by which Mfge8-expressing perivascular cells influence gastric antrum smooth muscle contractions? Investigating the signaling pathways and molecular interactions involved will be essential. Can Mfge8-based interventions be developed to treat or manage gastric motility disorders, such as gastroparesis or functional dyspepsia? Does Mfge8 play a broader role in gastrointestinal physiology, nutrient absorption, and metabolic health beyond its involvement in gastric motility? Can Mfge8 or its downstream targets be targeted pharmacologically to modulate gastric motility and potentially treat GI disorders? Can Mfge8 or its downstream targets be targeted pharmacologically to promote weight loss?
In a nutshell, the significant findings of Professor Brian Perrino and colleagues challenge our previous understanding of Mfge8’s distribution within the body and its role in the gastrointestinal system. It suggests that Mfge8-expressing perivascular cells play a key role in modulating gastric antrum smooth muscle contractions, potentially serving as a protective mechanism for the microcirculation. These findings have broader implications for our understanding of gastrointestinal physiology and may open doors to innovative therapeutic interventions for gastrointestinal disorders and metabolic health.
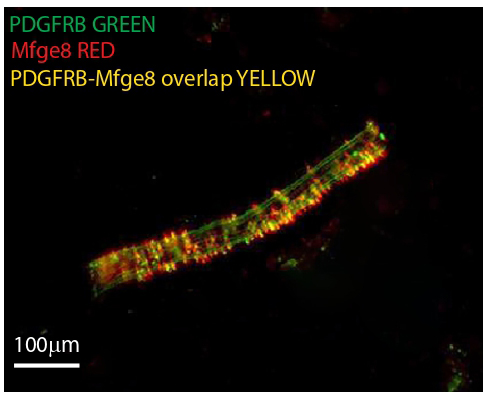
PDGFRB (GREEN) in a human gastric antrum submucosa
blood vessel using RNAscope in situ hybridization.
Reference
Perrino BA, Malogan J, Cobine CA, Sasse KC. Mfge8 is expressed by pericytes in gastric antrum submucosa from patients with obesity. Am J Physiol Cell Physiol. 2023 ;324(5):C992-C1006. doi: 10.1152/ajpcell.00043.2023.
 Medicine Innovates Medicine Innovates: Delivering innovations in medicine to the world for better health and prosperity
Medicine Innovates Medicine Innovates: Delivering innovations in medicine to the world for better health and prosperity

