Significance
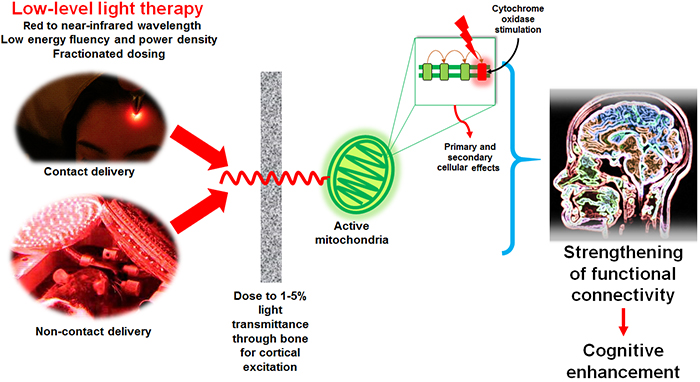
Cytochrome c oxidase (CCO) plays an important role in cellular energy metabolism, especially in the brain, because CCO catalyzes the final step in the mitochondrial electron transport chain, the reduction of oxygen to water. This process is coupled with the pumping of protons across the inner mitochondrial membrane, creating a proton gradient that drives the synthesis of adenosine triphosphate (ATP), the primary energy currency of the cell. Given the brain’s high demand for energy, efficient functioning of CCO is essential for maintaining neuronal activity and integrity. Changes in CCO activity can serve as a biomarker for the metabolic status of neurons and can indicate mitochondrial dysfunction, which is a feature of many neurodegenerative diseases, such as Alzheimer’s and Parkinson’s disease. Moreover, because of ts critical role in energy metabolism and brain function, CCO is a potential target for therapeutic interventions aimed at enhancing brain function or ameliorating the effects of neurological diseases. Techniques like photobiomodulation, which can influence CCO activity, are being explored for their potential benefits in various brain disorders.
To this account, a new study published in Frontiers in Neuroscience by Zachary Wade, Douglas Barrett, Roger Davis, Adrian Nguyen, Sindhu Venkat, and led by Professor F Gonzalez-Lima from the Department of Psychology and Institute for Neuroscience at the University of Texas at Austin, conducted a novel investigation to map how photobiomodulation influences CCO activity in the rat brain over time. The team applied transcranial infrared laser stimulation (TILS) to rats, targeting their brains with a specific wavelength (1064 nm) and power density (250 mW/cm²) for 50 seconds. This method aimed to non-invasively modulate CCO activity within the brain. They compared the brains of TILS-treated rats to those of control rats, which underwent a sham session without actual TILS. The authors collected brain samples at different post-TILS intervals: 1 day, 2 weeks, and 4 weeks, to assess the duration of photobiomodulation’s effects on CCO activity across various brain regions. The team used also quantitative enzyme histochemistry to analyze CCO activity in 28 specific regions of interest within the rat brains. Moreover, they quantifed changes in oxygen utilization which is a direct measure of CCO activity using digital imaging and densitometric analysis.
The authors observed that a single session of TILS could induce changes in CCO activity that persist for up to four weeks, indicating a long-lasting effect of photobiomodulation on brain bioenergetics. This suggests a sustained influence of photobiomodulation on brain bioenergetics, which could have far-reaching implications for clinical applications, particularly in cognitive enhancement and neurorehabilitation. They observed the earliest significant increase in CCO activity in the prefrontal infralimbic cortex post-TILS which highlights the potential of targeted photobiomodulation in modulating specific brain regions associated with cognitive functions. The persistence of elevated CCO activity in this region, as well as in others like the lateral septum and accumbens core, highlights the potential for photobiomodulation in promoting neuroplasticity and enhancing cognitive and emotional regulation. Beyond individual regions, the authors found significant correlations in CCO activity across different brain areas, especially four weeks post-TILS. This suggests that photobiomodulation may enhance functional connectivity within the brain, potentially influencing a wide range of neural processes and behaviors. However, they observed decrease in CCO activity in the medial amygdala at four weeks post-TILS which raises questions about the region-specific effects of photobiomodulation and the potential for varying responses based on brain region and function. This finding could suggest a nuanced balance of photobiomodulation effects, necessitating careful consideration of treatment parameters to achieve desired outcomes while avoiding potential adverse effects. It is noteworthy to mention that the study’s used quantitative enzyme histochemistry and digital imaging for analyzing CCO activity as an additional rigorous approach to elucidate the biochemical effects of photobiomodulation effects. This level of detail not only strengthens the findings but also sets a high standard for future research in the field.
The significant inter-regional CCO activity correlations observed post-TILS point to a broader impact of photobiomodulation on brain network connectivity. This aspect of the research has profound implications for understanding how photobiomodulation might influence complex brain functions and behaviors by modulating the activity of interconnected brain regions. The authors’ observation of different types of neuroplasticity changes at various time scales post-TILS, depending on the brain region and depth from the cortex, adds an important layer of complexity to our understanding of photobiomodulation’s mechanisms of action. In conclusion, the new study performed by Professor Gonzalez-Lima and his team is an important step forward to better understand the biological effects of photobiomodulation on the brain. The findings open new avenues for exploring the therapeutic potential of photobiomodulation in various neurological and psychiatric conditions. However, the complexity of the observed effects and the need for further research highlight the importance of a cautious approach to the clinical application of photobiomodulation. With further future research to investigate mechanisms underlying photobiomodulation’s effects on the brain, the potential for this non-invasive modality in enhancing brain health and function will become increasingly apparent.
Reference
Wade ZS, Barrett DW, Davis RE, Nguyen A, Venkat S, Gonzalez-Lima F. Histochemical mapping of the duration of action of photobiomodulation on cytochrome c oxidase in the rat brain. Front Neurosci. 2023;17:1243527. doi: 10.3389/fnins.2023.1243527.
 Medicine Innovates Medicine Innovates: Delivering innovations in medicine to the world for better health and prosperity
Medicine Innovates Medicine Innovates: Delivering innovations in medicine to the world for better health and prosperity

