Significance
Folate is an essential cofactor in one-carbon transfer reactions, playing a vital role in numerous biological processes across all domains of life. Folate biosynthesis is a complex and highly regulated pathway involving various enzymes and intermediates. In most organisms, the synthesis of p-aminobenzoate (pAB), a precursor to the folate cofactor, occurs through a well-established route. However, Chlamydia trachomatis, an obligate intracellular bacterial pathogen causing sexually transmitted infections and eye diseases, employs an alternative, non-canonical pathway for pAB biosynthesis. This pathway involves a unique protein known as CADD (chlamydia protein associating with death domains). CADD was initially identified as being involved in host cell apoptosis, but it was later discovered to possess pAB synthase activity. Despite significant interest, the mechanism and substrates of CADD-mediated pAB synthesis have remained unclear.
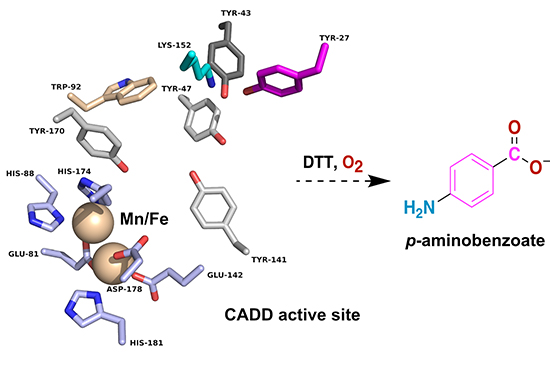
A recent study published in the peer-reviewed journal FEBS Letters, conducted by Rowan Wooldridge, Spenser Stone, Andrew Pedraza, W. Keith Ray, Richard Helm and Kylie Allen from Virginia Tech sheds light on the mechanism of CADD-dependent pAB synthesis. The researchers demonstrated that CADD could synthesize pAB in the presence of a reducing agent and molecular oxygen, without the addition of any exogenous substrates. Although other enzymes related to CADD utilize a diiron cofactor, further biochemical studies demonstrated that the pAB synthase activity of CADD is dependent on manganese and the physiological cofactor is likely a heterodinuclear Mn/Fe cluster.
The authors further investigated the reaction catalyzed by CADD and found that both oxygen atoms in the carboxylic acid portion of pAB are derived from molecular oxygen. This indicates that CADD functions as an oxygenase, with the oxygen incorporation potentially occurring at a site distant from the dimetal cofactor. The researchers also performed mass spectrometry analyses of CADD-derived peptides, which revealed that the enzyme employs its own amino acid residues as sacrificial substrates for pAB synthesis. Specifically, Tyr27 and Lys152 were identified as sacrificial aromatic and amino group donors, respectively.
The authors findings provide significant insights into the function of CADD and its unique role in pAB synthesis within C. trachomatis. This self-sacrificing mechanism, although seemingly wasteful, has been observed in other cofactor biosynthesis pathways and may not be detrimental to the organism since most cofactors are recycled.
C. trachomatis is a bacterium that can cause sexually transmitted infections. It is one of the most common STIs globally. It is highly prevalent, with millions of new cases reported each year worldwide. Early detection and treatment are crucial to prevent the spread of the infection. Untreated Chlamydia infections can lead to serious complications, both in men and women. Given its prevalence, potential for complications, and the importance of early detection and treatment, C. trachomatis remains a significant focus in medical research, public health initiatives, and sexually transmitted infections prevention efforts. The authors’ findings shed light on the mechanism of folate biosynthesis in C. trachomatis. Understanding the biosynthesis of folate, a crucial molecule involved in one-carbon transfer reactions, is essential for developing targeted therapies against Chlamydia infections.
The discovery of CADD’s metal dependence, particularly its reliance on manganese, has important implications for understanding the metal biology of C. trachomatis. This pathogen, like other successful pathogens, must overcome strict iron limitations imposed by the host. Thus, the use of of a Mn/Fe as opposed to a Fe/Fe cofactor by CADD may provide C. trachomatis an advantage in iron limiting conditions. Moreover, the study highlights the intriguing self-sacrificing mechanism employed by CADD, where amino acid residues within the enzyme act as substrates for pAB synthesis. This unconventional enzymatic behavior adds to our understanding of cofactor biosynthesis pathways and provides potential targets for future drug development.
Reference
Wooldridge R, Stone S, Pedraza A, Ray WK, Helm RF, Allen KD. The Chlamydia trachomatis p‐aminobenzoate synthase CADD is a manganese‐dependent oxygenase that uses its own amino acid residues as substrates. FEBS letters. 2023 ;597(4):557-72.
 Medicine Innovates Medicine Innovates: Delivering innovations in medicine to the world for better health and prosperity
Medicine Innovates Medicine Innovates: Delivering innovations in medicine to the world for better health and prosperity

