Significance
The quick and precise response of neutrophils to infections or tissue injuries is essential for human survival. These specialized immune cells employ a range of mechanisms to contain microbial threats. One of the most remarkable is the formation of neutrophil extracellular traps (NETs), web-like structures composed of genomic DNA, histones, and antimicrobial proteins that immobilize pathogens in the extracellular space. While NETs play a pivotal role in host defense, recent research has shown that, under certain conditions, they can also contribute to disease. Inappropriately formed NETs have been linked to a range of disorders, including lupus, rheumatoid arthritis, cystic fibrosis, and even metastatic cancers. Therefore, several research groups are actively exploring pharmacological strategies to inhibit the inappropriate formation of NETs. At the core of NETs formation is neutrophil elastase (NE). This serine protease enters the nucleus and dismantles histones, initiating chromatin decondensation. This decondensed DNA is mixed with cytoplasmic proteins and then released from the neutrophil, forming the structural backbone of NETs. Given its central role, NE has naturally emerged as a potential therapeutic target. However, developing specific and well-tolerated inhibitors has proven to be a challenging task. Many compounds affect other proteases or require toxic concentrations, which can interfere with broader immune functions. Among the body’s natural countermeasures to NE is secretory leukocyte protease inhibitor (SLPI), a small protein found in mucosal surfaces and body fluids that can suppress NE activity and dampen inflammation more broadly. However, its potential as a therapy has long been undercut by an ironic flaw: NE can cleave SLPI at its amino terminus, disarming it before it can exert its full effect. This feedback vulnerability severely limits the protein’s stability in inflammatory environments.
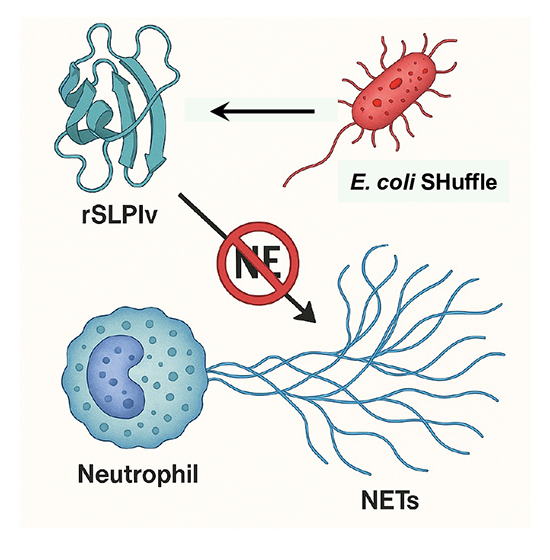
A new research paper, published in Protein Expression and Purification and conducted by Felipe Gonzalez Contreras, Roxana Gutierrez Vidal, and Xristo Zarate from the University of Nuevo Leon and Cinvestav in Mexico, reports that researchers re-engineered SLPI into a more robust form. Rather than reinvent the protein entirely, they took a minimalist yet strategic approach—substituting two amino acids at the N-terminal site most prone to NE cleavage. Their aim wasn’t just to protect SLPI from degradation but to preserve its function at lower, pharmacologically relevant doses. In doing so, they hoped to restore SLPI’s original promise—not as a blunt inhibitor, but as a precise modulator of inflammation during the critical early stages of immune activation. The authors began with a very targeted question: could they modify SLPI just enough to shield it from enzymatic inactivation without undermining its function? They focused on amino acids serine-15 and alanine-16, both of which are recognized as cleavage points for neutrophil elastase. Replacing them with glycine seemed, at least theoretically, to be a minor structural adjustment. But in proteins as compact and functionally sensitive as SLPI, even subtle edits can have unintended ripple effects. To test this SLPI variant (rSLPIv), they chose E. coli SHuffle T7 for expression—a system known for its capacity to form disulfide bonds in the cytoplasm, which is critical for a cysteine-rich protein like SLPI. They also opted to add an SmbP fusion tag, which wasn’t just for purification—it also helped with solubility, a notoriously problematic issue in prokaryotic expression. After a round of optimization, the results were solid. Electrophoretic analysis revealed a clean band and mass spectrometry confirmed the expected molecular weight. But all of this was, in essence, a preamble. The real test came when they exposed freshly isolated human neutrophils to PMA, a known trigger of NETosis that can lead to the formation of large nuclear areas (>800 µm²), and then added rSLPIv at different concentrations. What stood out immediately was the potency: 10 nM was sufficient to reduce NETs formation by approximately 30%. That’s a meaningful reduction—especially given that wild-type SLPI often requires micromolar dosing to achieve a comparable effect. More surprisingly, the suppression held over several hours. Such durability is rare in this field. Following this, the authors used confocal microscopy, where they stained the DNA and looked at nuclear morphology, measuring the spread of the nuclei. As expected, they found that PMA alone caused the nuclei to swell and unravel—hallmarks of chromatin decondensation. However, when treated with rSLPIv, particularly at a concentration of 50 nM, the nuclei remained tightly packed and much more compact. Quantitative image analysis supported this observation: over half of the cells (69%) displayed a smaller nuclear area, comparable to that of neutrophils not exposed to PMA, with sizes below 800 µm².
The scope of this work reaches far beyond the mechanistic details of NETs biology. While NETosis was once thought of as an isolated immune event, it has since been implicated in a staggering variety of pathological contexts—autoimmune diseases like lupus and rheumatoid arthritis, of course, but also sepsis, cancer metastasis, and most recently, the thromboinflammatory damage seen in severe COVID-19 cases. Despite this growing body of evidence, therapeutic strategies to specifically inhibit NETs formation remain frustratingly limited. At present, DNase I is the only FDA-approved agent that targets NETs, and even then, it does so only after they’ve formed. By degrading the DNA backbone, it clears the structural web. Still, it leaves behind enzymatically active proteases that can exacerbate tissue damage—hardly a perfect solution. That’s where rSLPIv offers a compelling alternative. Rather than cleaning up after the fact, it prevents NETs from forming in the first place—intervening at the level of chromatin decondensation upstream of DNA extrusion. Mechanistically, this is a far cleaner point of control. From a clinical standpoint, it may also be safer as it avoids triggering a secondary wave of inflammatory damage caused by the release of proteases, such as neutrophil elastase.
Equally worth noting is the platform used to produce the molecule. Recombinant expression of human proteins in E. coli is often viewed as a compromise, especially for proteins that are small, rich in disulfide bonds, or structurally delicate—rSLPIv ticks all three boxes. However, in this case, Dr. Xristo Zarate and colleagues successfully produced a high-purity, soluble variant using a relatively straightforward bacterial system, which highlights the elegance of their construct design. This has real implications for scalability and cost—two factors that routinely derail biologics in early translational stages. Moreover, minimalistic, structure-informed engineering can breathe new life into endogenous proteins that might otherwise be dismissed as therapeutically impractical. With a focus on N-terminal cleavage, they successfully enhanced molecular durability while preserving SLPI’s innate regulatory function.
Dr. Xristo Zarate, Principal Investigator, told Medicine Innovates: “This project is a clear demonstration of how biotechnology and protein engineering can converge to solve complex problems in immune regulation. It also reflects the dedication of young scientists like Felipe, whose work is shaping the next generation of therapeutic proteins“
Reference
Felipe de Jesus Gonzalez-Contreras, Roxana Guadalupe Gutierrez-Vidal, Xristo Zarate, A recombinant human SLPI variant suppresses the formation of neutrophil extracellular traps at low concentrations in vitro, Protein Expression and Purification, Volume 232, 2025, 106721,
 Medicine Innovates Medicine Innovates: Delivering innovations in medicine to the world for better health and prosperity
Medicine Innovates Medicine Innovates: Delivering innovations in medicine to the world for better health and prosperity



