Significance
Glioblastoma is an aggressive form of brain cancer characterized by rapid tumor growth, invasion of nearby tissues, and resistance to conventional treatments. Targeting tumor cell metabolism has emerged as a promising approach to treat glioblastoma by exploiting the unique metabolic features of cancer cells. In this response, I will explain in detail how targeting tumor cell metabolism can be used to treat glioblastoma. Glioblastoma cells exhibit several metabolic alterations compared to normal brain cells. These alterations include increased glucose uptake, enhanced glycolysis (the breakdown of glucose), and altered mitochondrial metabolism. These changes support the increased energy demands of rapidly dividing cancer cells and contribute to their survival and growth.
The “Warburg effect” is a phenomenon observed in many cancer cells, including glioblastoma, where cancer cells preferentially use glycolysis for energy production even in the presence of oxygen (aerobic glycolysis). This metabolic switch allows cancer cells to generate energy and metabolic intermediates needed for cell growth and proliferation. Targeting this metabolic reprogramming presents an opportunity for therapeutic intervention. One approach to targeting tumor cell metabolism in glioblastoma is to inhibit glucose metabolism. This can be achieved by using small molecule inhibitors that target key enzymes involved in glycolysis. For example, inhibitors of enzymes like hexokinase 2 (HK2), which initiates the first step of glucose metabolism, have shown promise in preclinical studies. By blocking glucose metabolism, cancer cells are deprived of the energy and building blocks needed for their survival.
Another strategy to target glioblastoma metabolism involves inhibiting mitochondrial function. Mitochondria play a crucial role in energy production and cellular metabolism. In cancer cells, mitochondrial metabolism is often dysregulated, leading to increased reliance on glycolysis. Targeting mitochondrial metabolism can disrupt the energy balance and induce cell death. Small molecules that target specific mitochondrial proteins or disrupt mitochondrial membrane potential have shown efficacy in preclinical models of glioblastoma.
Given the complexity and heterogeneity of glioblastoma, a single-target approach may not be sufficient. Combination therapies that target multiple metabolic pathways have shown promise. Combining inhibitors of glycolysis or mitochondrial metabolism with other standard treatments like radiation or chemotherapy can enhance their effectiveness and overcome resistance.
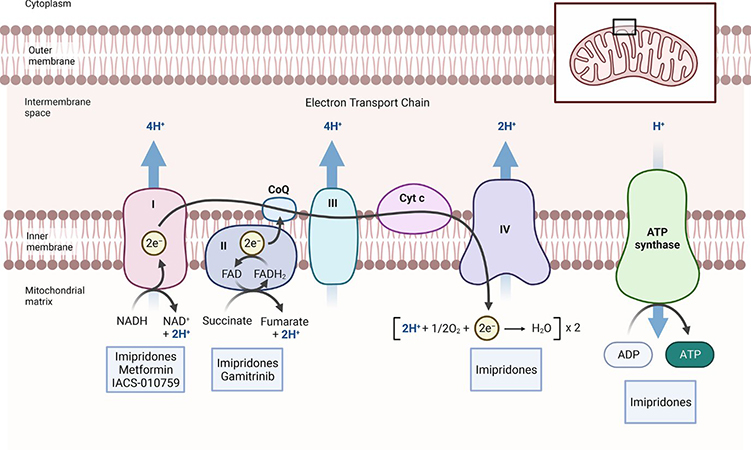
The oxidation of NADH2 and FADH2 are fundamental since NAD and FAD are the key components of the TCA-cycle that is critical to entertain biosynthesis in cancer cells. The TCA-cycle itself is predominantly fueled through carbons from glucose, glutamine, fatty acids and lactate. Targeting mitochondrial energy metabolism appears feasible through several drug compounds that activate the CLPP protein or interfere with NADH-dehydrogenase, pyruvate-dehydrogenase, enzymes of the TCA-cycle and mitochondrial matrix chaperones. While these compounds have demonstrated anti-cancer effects in vivo, recent research suggests which patients most likely benefit from such treatments.
While glycolysis is abundant in malignancies, mitochondrial metabolism is significant as well. Mitochondria harbor the enzymes relevant for cellular respiration, which is a critical pathway for both regeneration of reduction equivalents and energy production in the form of ATP.
In a new study published in the peer-reviewed journal, Oncotarget, Enyuan Shang, Trang Thi Thu Nguyen, Mike-Andrew Westhoff, Georg Karpel-Massler, and led by professor Markus D. Siegelin from Columbia University Medical Center, City University of New York and Ulm University Medical Center discussed their recent finding that FDA-approved HDAC-inhibitors may have a profound impact on energy metabolism in solid tumor cells, including glioblastoma (GBM).
Because of the impact of HDAC-inhibitors on metabolism the authors hypothesized that imipridones, which suppress cellular respiration, might synergize with these compounds to significantly enhance killing of GBM cells. Indeed, they found that imipridones reversed HDAC-inhibitor induced activation of cellular respiration and in turn the combination treatment facilitated induction of intrinsic apoptosis in a manner that was partially reliant on the anti-apoptotic Bcl-2 family members.
In summary, the authors demonstrated that targeting tumor cell metabolism is a viable therapeutic strategy for treating glioblastoma. By exploiting the unique metabolic features of cancer cells, such as increased glucose uptake, altered mitochondrial metabolism, and dysregulated amino acid metabolism, researchers are developing novel therapies to disrupt cancer cell growth and survival. These approaches offer the potential to improve the outcomes for patients with glioblastoma, although further research and clinical trials are needed to establish their efficacy and safety.
Reference
Shang E, Nguyen TTT, Westhoff MA, Karpel-Massler G, Siegelin MD. Targeting cellular respiration as a therapeutic strategy in glioblastoma. Oncotarget. 2023 ;14:419-425. doi: 10.18632/oncotarget.28424.
 Medicine Innovates Medicine Innovates: Delivering innovations in medicine to the world for better health and prosperity
Medicine Innovates Medicine Innovates: Delivering innovations in medicine to the world for better health and prosperity

